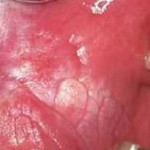
Oral leukoplakia is a white patch found on the oral mucosa that cannot be rubbed off. The term ‘leukoplakia’ should be used when other oral mucosal conditions presenting as white lesions (e.g. frictional keratosis, lichen planus, white sponge nevus, hairy leukoplakia) have been excluded. The prevalence in the general population ranges between 1-5%. Leukoplakia is one of a group of potentially malignant disorders with a rates of malignant transformation reported to range from 0-36%
The aim of this Cochrane review is to assess the effectiveness, safety and acceptability of treatments for leukoplakia in preventing oral cancer.
Methods
Searches were conducted in the Cochrane Oral Health’s Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) Medline, Embase, CancerLit, the metaRegister of Controlled Trials, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform. There were no restrictions on the language or date of publication.
Randomised controlled trials (RCTs) enrolling people with a diagnosis of oral leukoplakia and comparing any treatment versus placebo or no treatment were considered. The primary outcome was oral cancer development with clinical resolution, improvement of histologically features and adverse effects as secondary outcomes.
Two reviewers independently selected studies and abstracted data with study quality being assessed using the Cochrane risk of bias tool with the overall quality of evidence being assessed using the GRADE criteria. Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated for dichotomous outcomes and meta-analysis were conducted when valid and relevant data were available.
Results
- 14 studies involving a total of 909 patients were included.
- 6 studies were considered to be at high risk of bias, 7 at unclear risk and only 1 at low risk.
- There were no trials of surgical treatments that have been assessed in RCTs that include a no treatment or placebo comparison.
- 4 studies looked at vitamin A and retinoids; 3 studies at beta carotene or carotenoids, 2 studies at the non- steroidal anti-inflammatory drugs (NSAIDs), ketorolac and celecoxib; 4 studies at herbal extracts, including tea components, a Chinese herbal mixture and freeze-dried black raspberry gel; 1 study looked at bleomycin study); and 1 at Bowman-Birk inhibitor.
- Of 5 studies reporting cancer incidence only 3 provided useable data
- None of the studies provided evidence that active treatment reduced the risk of oral cancer more than placebo:
- Systemic vitamin A (1 study, 85 patients) RR = 0.11(95%CI; 0.01 to 2.05).
- Systemic beta carotene (2 studies, 132 patients) RR= 0.71 (95%CI; 0.24 to 2.09).
- Topical bleomycin (1 study, 20 patients) RR= 3.00, (95%CI; 0.32 to 27.83).
- Follow-up ranged between two and seven years.
- Some individual studies suggested effectiveness of some proposed treatments, namely, systemic vitamin A, beta carotene and lycopene, for achieving clinical resolution of lesions more often than placebo.
- Single studies found that systemic retinoic acid and lycopene may provide some benefit in terms of improvement in histological features.
- Some studies reported a high rate of relapse.
- Side effects of varying severity were often described; however, it seems likely that interventions were well accepted by participants because drop-out rates were similar between treatment and control groups.
Conclusions
The authors concluded:-
Surgical treatment for oral leukoplakia has not been assessed in an RCT that included a no treatment or placebo comparison. Nor has cessation of risk factors such as smoking been assessed. The available evidence on medical and complementary interventions for treating people with leukoplakia is very limited. We do not currently have evidence of a treatment that is effective for preventing the development of oral cancer. Treatments such as vitamin A and beta carotene may be effective in healing oral lesions, but relapses and adverse effects are common. Larger trials of longer duration are required to properly evaluate the effects of leukoplakia treatments on the risk of developing oral cancer. High-quality research is particularly needed to assess surgical treatment and to assess the effects of risk factor cessation in people with leukoplakia.
Comments
Leukoplakia is the commonest of the potentially malignant oral disorders. Yet this review highlights the paucity of high quality RCT evidence available for its treatment. The study with the longest duration in this review is 7 years (with only 4 studies being longer than 12 months) but as the authors highlight in their discussion fewer than half of those with leukoplakia develop oral cancer within two years of diagnosis and incidence of oral cancer increase with the duration of follow up of these patients. This means that we are going to require undertaking large multi-centre RCTs that assess outcomes after 10 years to see the impact of treatments on cancer incidence. The other disappointing finding in this review is that despite a clinical preference for leukoplakia to be treated surgical no RCTs have been conducted and the only information is from observational studies.
Links
Primary paper
Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, Carrassi A, MacDonald LCI, Worthington HV. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No.: CD001829.

Oral leukoplakia: very limited evidence for treatment https://t.co/ss5gvlujGd
Review found no trials for surgical treatments of oral leukoplakia https://t.co/ss5gvlujGd
Very limited evidence for treatments of oral leukoplakia https://t.co/HE4bDUsZbH
Very limited evidence for treatments of oral leukoplakia https://t.co/ss5gvlujGd
Evidence on medical & complementary interventions for treating leukoplakia is very limited https://t.co/rJgs8eHupL
Evidence on medical & complementary interventions for treating leukoplakia is very limited https://t.co/ss5gvlujGd
Don’t miss-Oral leukoplakia: very limited evidence for treatments https://t.co/ss5gvlujGd