
Depression affects an estimated 300 million people worldwide (WHO, 2017) and is associated with impaired cognitive processing and poor ability to regulate negative emotions and thoughts (Gotlib & Joorman, 2010). Depression can be either unipolar, also known as major depressive disorder (MDD), or bipolar, a form in which depressive episodes alternate with manic episodes and normal mood.
The high prevalence of depression and its adverse effects on quality of life have mobilised researchers and clinicians to explore a wide array of treatments targeting this psychiatric disability. Whilst first-line treatments, such as antidepressant medication and talking therapies, have proved useful to some individuals, approximately 50% of patients do not improve following these interventions (Cuijpers et al., 2020). McClintock and colleagues (2020) addressed this need for novel treatment approaches by investigating the effectiveness of repeated exposure to transcranial direct current stimulation (tDCS) in unipolar and bipolar depression.
Antidepressants and talking therapies are useful for some people with depression, but about half of us do not get better with these treatments.
Methods
The authors conducted a triple-blind randomised controlled trial on 130 participants across six academic medical centres in Australia and the United States. Participants were randomly assigned to 20 sessions of tDCS at a low dose (0.034 mA for 30 min; realistic sham stimulation) or high dose (2.5 mA for 30 min) over four weeks (randomised phase). Randomisation was achieved through a computer-generated random number sequence (Alonzo et al., 2016), thus eliminating systematic bias. Afterwards, all participants could choose whether to engage in a 4-week open-label high dose treatment (open-label phase).
The tDCS protocol was presented in great detail. The anode was placed over the left dorsolateral prefrontal cortex at F3, an area supporting working memory and cognitive control, whereas the cathode was placed at F8, a region important for emotion regulation.
Neurocognitive and clinical assessments were taken at baseline, after the randomised phase and upon completion of the open-label phase. The neuropsychological battery assessed verbal learning and memory, selective attention, auditory attention and working memory, psychomotor processing speed and visuospatial attention, and subjective cognitive functioning. Mood was assessed using the MADSR (Montgomery & Asberg, 1979), a depression scale designed to be sensitive to treatment-related changes in mood.
DNA was extracted through a saliva or blood sample to allow the genetic analysis of the BDNF Val66Met (rs6265) and the COMT Val108/158Met (rs4680) single nucleotide polymorphisms (SNPs), which have been shown to moderate tDCS effects on neurocognitive performance in the past (Nieratschker et al., 2015).
Results
Participants in the low tDCS and high tDCS groups did not differ significantly on demographic and clinical outcomes. A mixed-effects analysis was used to investigate tDCS-related changes in neuropsychological functioning following the study. These analyses were completed separately for unipolar and bipolar patients. There were no group nor time x group effects on neuropsychological functioning for either group. However, unipolar and bipolar patients presented an improved performance on measures of verbal learning and recall, selective attention, information processing speed, and working memory over time.
Furthermore, BDNF Val66Met and COMT Val158Met polymorphisms interacted with tDCS dose, such that Val/Val carriers in the high dose group performed significantly better on measures of verbal learning and memory than those in the low dose group, whereas COMT Val158Met carriers in the high dose group performed worse on phonemic fluency tasks compared to carriers in the low dose group.
Effect sizes were reported for genetic analyses but not for neuropsychological assessments. Adverse effects and harms were reported for both the low dose and high dose groups, with very few participants experiencing visual or gastrointestinal side effects, headaches and allergic reactions to the headband. Nonetheless, there was no differential drop-out between the study groups.

The study suggested that both patients with unipolar and bipolar depression presented an improved performance on verbal learning and recall, selective attention, information processing speed, and working memory over time.
Conclusions
The authors concluded that tDCS may enhance cognitive performance in unipolar and bipolar patients at low and high doses, independently of the antidepressant effects of the intervention. These effects seem to be moderated by genotype, such that Val/Val carriers seem to have benefited more from a high tDCS dose in what concerns their verbal learning and memory, whereas COMT Val158Met carriers presented an improved phonemic fluency in the low dose rather than high dose group.
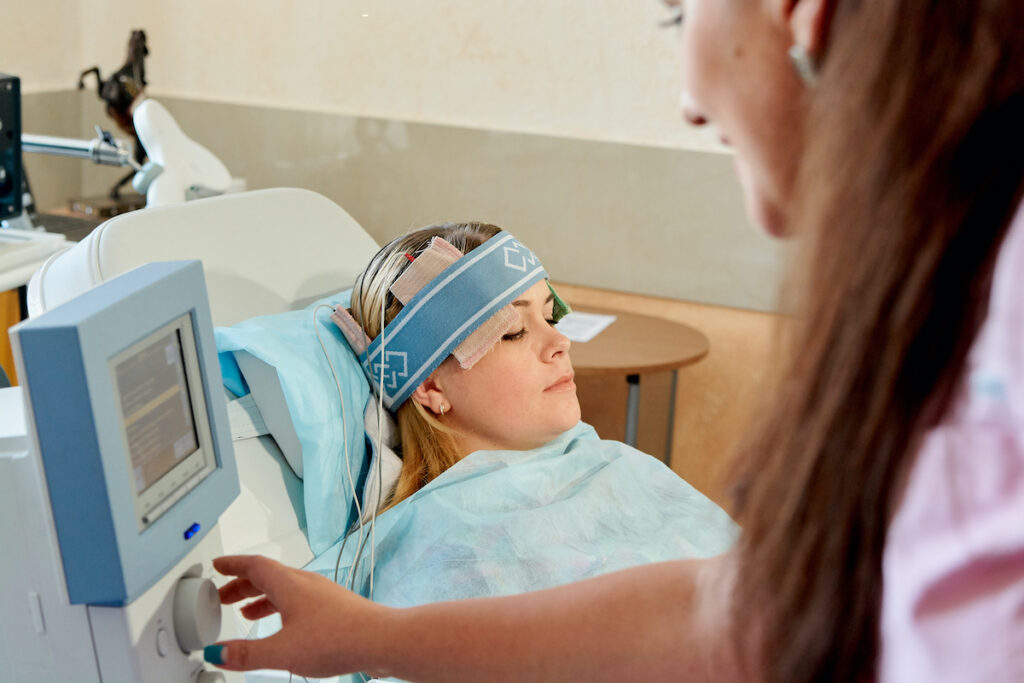
tDCS improved neuropsychological functioning in unipolar and bipolar patients irrespective of dose or mood outcomes. However, the neuropsychological effects of tDCS are moderated by genotype.
Strengths and limitations
The current study has many strengths, as follows:
- It was a triple-masked study, meaning that there was low risk of bias or placebo effects affecting neurocognitive or clinical outcomes.
- Baseline clinical and demographic characteristics were clearly set out for each group and there were no between-group differences that could have affected the outcomes of the trial.
- The study protocol was rigorously defined and a CONSORT Flow Diagram accounted for participant losses and follow-up exclusion.
- Sample size sufficiently large to support the reliability of the current findings.
The study also had a few limitations, as there was no true sham stimulation condition, making it difficult to rule out placebo or practice effects. Nonetheless, the main limitation of this study is that, based on the mixed effect analysis, the authors concluded that low and high doses of tDCS lead to similar neuropsychological outcomes. However, participants in the low dose group completed 4 weeks of low dose stimulation which was then followed by 4 weeks of high dose stimulation, therefore there is no participant group that completed 8 weeks of low dose tDCS. Therefore, based on the current study we can only conclude that combining 4 weeks of low dose and 4 weeks of high dose stimulation leads to similar effects as an 8-week course of high dose stimulation. The only way in which we could conclude that tDCS at such a low dose (0.034 mA) is biologically active is by employing EEG to measure its offline and online effects across time.
Finally, the authors of the study could have conducted a cost-effectiveness analysis to determine whether the effectiveness of tDCS for neuropsychological functioning justifies its high cost compared to existing first-line psychological and pharmacological interventions.

The current study followed a rigorous protocol described in sufficient detail, while the triple-mask blinding secured that clinical and neurocognitive outcomes wouldn’t be affected by bias or placebo effects.
Implications for practice
The current findings indicate that DNA analyses could be used to predict whether individuals would benefit more from a low or a high dose of tDCS, thus paving the way towards a future of personalised interventions. As transcranial stimulation is already used by the NHS to treat depression, genetic analyses could save time and resources by assigning people to the intervention predicted to be most useful according to their genetic or neuropsychological profile. Furthermore, future studies could combine tDCS with neuroimaging methods to identify the levels within which non-invasive brain stimulation is biologically active and leads to the lowest number of side effects.
As a person with lived experience of depression, I know how exhausting it is to even ask for help, and how demoralising it feels to be assigned to a treatment that does not work for you. This is why I strongly believe that there is an acute need for personalised treatments, an approach that has proved effective in my own research on individual differences that predict cognitive and affective outcomes following working memory training. Therefore, I am hopeful that the current study will bring hope and inspiration to researchers, practitioners and people with lived experience of depression, and that together we can make sure that every person can benefit from the intervention most suitable to their neurocognitive and genetic profile.

The current findings pave the way towards a future in which genetic analyses allow for personalised treatment approaches in depression.
Statement of interests
No conflicts of interest.
Links
Primary paper
McClintock, S. M., Martin, D. M., Lisanby, S. H., Alonzo, A., McDonald, W. M., Aaronson, S. T., … & Loo, C. K. (2020). Neurocognitive effects of transcranial direct current stimulation (tDCS) in unipolar and bipolar depression: Findings from an international randomized controlled trial. Depression and Anxiety, 37, 261-272.
Other references
Alonzo, A., Aaronson, S., Bikson, M., Husain, M., Lisanby, S., Martin, D., … & Loo, C. (2016). Study design and methodology for a multicentre, randomised controlled trial of transcranial direct current stimulation as a treatment for unipolar and bipolar depression. Contemporary Clinical Trials, 51, 65-71.
Cuijpers, P., Stringaris, A., & Wolpert, M. (2020). Treatment outcomes for depression: Challenges and opportunities. The Lancet Psychiatry, 7, 925-927.
Gotlib, I. H., & Joormann, J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285-312.
Montgomery, S. A., & Åsberg, M. (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382-389.
Nieratschker, V., Kiefer, C., Giel, K., Kruger, R., & Plewania, C. (2015). The COMT Val/Met polymorphism modulates effects of tDCS on response inhibition. Brain Stimulation, 8, 283–288.
World Health Organization. (2017). Depression and other common mental disorders: Global health estimates (No. WHO/MSD/MER/2017.2). World Health Organization.
Photo credits
- Photo by Adam Nieścioruk on Unsplash

I long for the day of personalised treatments, and it gives me hope for the DNA analysis prediction. As I’m in no way medical, I was unsure if they gave the treatment in conjunction with bipolar medicine or if it was a standalone process.