
When Roland Kuhn reported efficacy for the first antidepressant, imipramine, in a 1957 paper, he included observation of Abstinenzerscheinungen; withdrawal symptoms. Hundreds of journal articles have since been published describing antidepressant withdrawal symptoms in adults, teens, children, and neonates. Recently, belief that antidepressant withdrawal syndrome is infrequent, mild, and short-lived has been challenged by evidence that incidence, severity, and duration are much greater than presumed. Indications are that 40% or more of those coming off antidepressants will experience unpleasant withdrawal symptoms.
In 2019, antidepressant use was estimated at 17% of adults in England, with similar rates in other developed economies, a high proportion of prescriptions without evidence-based indication. Pharmaceutical industry watchers think the 2020 global antidepressants market doubled that of 2019, harvesting $28.6 billion compared to $14.3 billion, due to pandemic-related increase in demand.
The zeitgeist is psychotropic. A new question for medicine: how to minimise the risk of psychotropic withdrawal symptoms, has become vitally important to tens of millions of people. Many taking antidepressants eventually will want or need to come off, for good reasons. What is the best way for them to discontinue, with the lowest risk of withdrawal symptoms or relapse?
To answer that question, a team from the Cochrane Common Mental Disorders group conducted a systematic review of studies where antidepressants were taken for 6 months or more and then discontinued (Van Leeuwen et al, 2021).
Many millions are taking antidepressants, while the global antidepressants market possibly doubled in 2020 due to pandemic stress. How will all these people come off their drugs?
Methods
Led by Ellen Van Leeuwen, a primary care doctor in Belgium, the Cochrane Common Mental Disorders Group reviewers searched international databases for randomised controlled trials (RCTs) of any design prior to April 2020, published and unpublished, that utilised discontinuation approaches and compared results with continuation of antidepressants (or usual care) for people over the age of 18 with depression or anxiety, prescribed any class of antidepressant at any dosage for at least six months (Van Leeuwen et al, 2021).
Excluded were cross-over trials; those that included participants with a history of bipolar disorder, obsessive-compulsive disorder, or psychosis; or discontinuation trials in the context of electroconvulsive therapy or hospital admission.
Discontinuation approaches may be abrupt stoppage (including substitution of placebo) or tapering of the drug. For either discontinuation approach, the Cochrane group also assessed effectiveness of any accompanying informal or formal psychological or psychosocial intervention or supplemental education of the treating physician.
The group sought but could not find any studies that included pharmacological aids to discontinuation, such as use of another form of the drug, e.g. liquid paroxetine or fluoxetine, to facilitate gradual tapering, or short-term benzodiazepine treatment for withdrawal symptoms.
Outcomes
The study defined primary outcomes as
- Successful discontinuation rate
- Relapse (as defined by authors of the original study)
- Withdrawal symptoms
- Adverse events
Secondary outcomes were depressive symptoms, anxiety symptoms, quality of life, social and occupational functioning, and severity of illness.
Results
The group identified 33 studies, which included a total of 4,995 subjects. Relapse rather than discontinuation was the primary outcome for 31 studies. Only one study reported data on withdrawal symptoms. In reporting their results, the Cochrane authors offer this caveat:
All included trials were at high risk of bias. The main limitation of the review is bias due to confounding withdrawal symptoms with symptoms of relapse of depression. Withdrawal symptoms (such as low mood, dizziness) may have an effect on almost every outcome including adverse events, quality of life, social functioning, and severity of illness.
Due to this fatal flaw, the Cochrane group was unable to conclude whether any of the discontinuation strategies were safe and effective, further noting none employed tapering schemes beyond a few weeks.
Overall, the most solid finding was an absence of convincing evidence in every dimension:
We found the certainty of evidence for primary and secondary outcomes to be very low to low due to limitations in study design, indirectness, and imprecision.
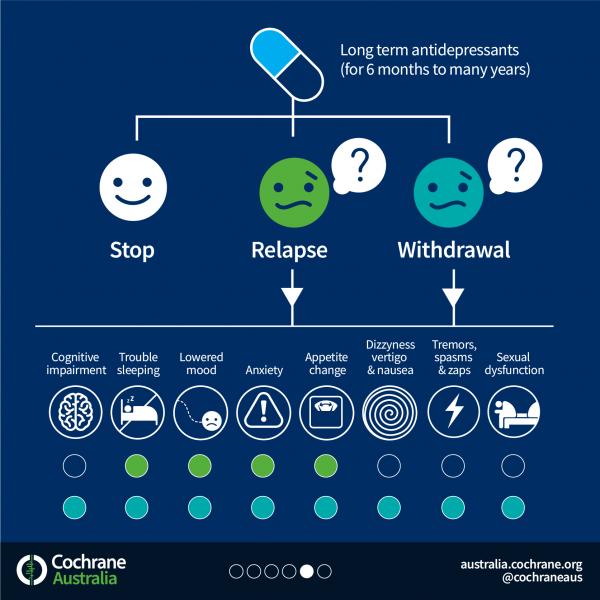
“The main limitation of the review is bias due to confounding withdrawal symptoms with symptoms of relapse of depression.” (Van Leeuwen et al, 2021)
Conclusions
Viewing the very low to low certainty and paucity of data derived from a highly representative evidence base regarding antidepressant discontinuation, the authors soberly concluded:
We cannot make any firm conclusions about effects and safety of the approaches studied to date. The true effect and safety are likely to be substantially different from the data presented due to assessment of relapse of depression that is confounded by withdrawal symptoms. All other outcomes are confounded with withdrawal symptoms.
They observed there might not be much difference in risk of withdrawal symptoms between abrupt discontinuation and tapers of ≤ 4 weeks because the latter was inadequate.
Further:
There is an urgent need for trials that adequately address withdrawal confounding bias, and carefully distinguish relapse from withdrawal symptoms. Future studies should report key outcomes such as successful discontinuation rate and should include populations with one or no prior depression episodes in primary care, older people, and people taking antidepressants for anxiety and use tapering schemes longer than 4 weeks.

This Cochrane review concludes that little is known about safe ways to come off antidepressants. The reviewers call for studies that recognise withdrawal syndrome and investigate effective tapering strategies for the millions of people on antidepressants.
Strengths and limitations
In search of reliable evidence, this very comprehensive 186-page investigation of the antidepressant discontinuation study domain painstakingly combed heterogenous RCTs that reported results in inconsistent ways and often failed to capture important data.
The authors were compelled to downgrade all studies due to various biases, the most pervasive being lack of protocols to distinguish withdrawal symptoms from relapse. Withdrawal symptoms should have been an expected outcome of antidepressant discontinuation. All but one of the 33 studies reviewed were looking for rate of relapse after discontinuation rather than perhaps a mix of relapse and withdrawal symptoms; not surprisingly, they found only a high risk of relapse.
It is unclear what happened to those people after they went off their drugs.
The Cochrane authors also doubt the evidence base for antidepressant continuation, as it depends on the same and similar studies thoroughly confounded by withdrawal, which is probably mistaken for relapse.
As the authors put it:
Consequently, it is unclear to what degree misclassified withdrawal symptoms contributed to “relapse” rates. Research suggests this could pertain to most relapses (El-Mallakh 2012; Greenhouse 1991; Hengartner 2020; Recalt 2019; Rosenbaum 1988). Moreover, withdrawal symptoms may have an effect on almost every outcome including adverse events, quality of life, social functioning, severity of illness, and anxiety and depression scores. For example, low mood and other withdrawal symptoms may register on the Hamilton Rating Scale for Depression (HAM-D) – the prioritised measure for depressive symptoms – and may result in people falsely allocated to having “severe” depressive symptoms.
The authors also observed the subjects in 25 of the studies had “recurrent” or chronic depression not representative of the majority of the general public taking antidepressants.
Despite tabulating many comparisons of discontinuation approach permutations, in the miasma of confound, the authors could not produce a comparison of abrupt versus “tapered” discontinuation. The sole study that reported data on withdrawal symptoms (Khan, 2014) also was the only one that directly compared outcomes of abrupt versus “tapered” discontinuation, finding no difference between them. Pfizer conducted it to show the FDA a range of desvenlafaxine dosages was unnecessary for tapering. Cochrane assigned it high “other” bias.
From their deep familiarity with the material, the authors surmised that low-risk antidepressant discontinuation may take longer than ≤4 weeks and require different techniques than those employed so far, potentially gradual hyperbolic tapering over months as outlined in Horowitz, 2019.

With unrecognised withdrawal symptoms confounding the evidence base of antidepressant discontinuation, we cannot assume that what happens when people go off antidepressants is relapse. Therefore, justification for continuation may be shaky.
Implications for practice
Until very recently, initiation and continuation have been the primary focus of antidepressant prescribing, with little thought about the best ways to come off the drugs or consideration of withdrawal effects in risk-benefit assessment. Informed by the evidence base represented by these RCTs, clinicians have expected relapse after discontinuation of antidepressants, and that continuation, possibly long-term, was better for patients than going off.
Based on this review, the Cochrane review authors (Van Leeuwen et al, 2021) advise clinicians that:
- Because of confounds, the evidence is unreliable for either discontinuation approach or risk of relapse after discontinuation.
- It is unclear how long antidepressant treatment has to be maintained after remission. Current guidelines are based on consensus rather than evidence.
- Evidence is lacking for appropriate discontinuation approaches for those who do not have “recurrent” depression, the elderly, and those taking antidepressants for anxiety.
- The effect of short tapering regimens (≤ 4 weeks) was similar to abrupt discontinuation. Clinicians should expect to taper much slower, perhaps using liquid drug forms or tapering strips, while closely monitoring for withdrawal symptoms.
- To taper effectively, clinicians will need to recognise withdrawal symptoms. Withdrawal symptoms differ from relapse or recurrence in timing of onset (within days rather than weeks), a rapid reversal after reintroduction of the antidepressant, and the emergence of somatic and psychological symptoms different from the original illness (e.g. shock-like sensations, dizziness, pronounced insomnia). Utilising the Discontinuation-Emergent Signs and Symptoms (DESS) Scale (PDF) may be helpful in monitoring reductions in dosage. When the patient’s DESS score returns to baseline after a reduction, further reduction is appropriate.

This Cochrane review questions the evidence base, which shows that patients are far better off staying on antidepressants.
Statement of interests
Adele Framer: Under the pseudonym Altostrata, I am the founder and administrator of SurvivingAntidepressants.org, a Web community of 15,000 members offering tapering information and volunteer peer support for withdrawal and protracted withdrawal syndrome from psychiatric drugs. Donations underwrite site expenses. I do not receive any compensation, monetary or otherwise, for serving in this capacity. I have coauthored papers with Mark Horowitz (who is a coauthor on this Cochrane review) and I am a member of the International Institute for Psychiatric Drug Withdrawal.
Links
Primary paper
Van Leeuwen, E., van Driel, M., De Sutter, A., Robertson, L., Kendrick, T., Horowitz, M., Donald, M., & Christiaens, T. (2021). Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults (Review). Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD013495.pub2
Other references
Davies, J., & Read, J. (2019). A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: Are guidelines evidence-based? Addictive Behaviors, S0306460318308347. https://doi.org/10.1016/j.addbeh.2018.08.027
Framer, A. (2021). What I have learnt from helping thousands of people to taper off antidepressants and other psychotropic medications. Therapeutic Advances in Psychopharmacology. https://doi.org/10.1177/2045125321991274
Groot, P. C., & van Os, J. (2020). Outcome of antidepressant drug discontinuation with taperingstrips after 1–5 years. Therapeutic Advances in Psychopharmacology, 10, 204512532095460. https://doi.org/10.1177/2045125320954609
Hengartner, M. P., Schulthess, L., Sorensen, A., & Framer, A. (2020). Protracted withdrawal syndrome after stopping antidepressants: A descriptive quantitative analysis of consumer narratives from a large internet forum. Therapeutic Advances in Psychopharmacology. https://doi.org/10.1177/2045125320980573
Horowitz, M. A., & Taylor, D. (2019). Tapering of SSRI treatment to mitigate withdrawal symptoms. The Lancet Psychiatry, 6(6), 538–546. https://doi.org/10.1016/S2215-0366(19)30032-X
Jauhar, S., & Hayes, J. (2019). The war on antidepressants: What we can, and can’t conclude, from the systematic review of antidepressant withdrawal effects by Davies and Read. Addictive Behaviors, 97, 122–125. https://doi.org/10.1016/j.addbeh.2019.01.025
Khan, A., Musgnung, J., Ramey, T., Messig, M., Buckley, G., & Ninan, P. T. (2014). Abrupt Discontinuation Compared With a 1-Week Taper Regimen in Depressed Outpatients Treated for 24 Weeks With Desvenlafaxine 50 mg/d. Journal of Clinical Psychopharmacology, 34(3), 365–368. https://doi.org/10.1097/JCP.0000000000000100
Kuhn, R. (1957). On the treatment of depressive states of an iminodibenzl derivative (G22355). Schweizerische Medizinische Wochenschrift, 87(35–36), 1135–1140.
Pratt, J. A., Brody, D. J., & Gu, Q. (2017). Antidepressant use among persons aged 12 and over: United States, 2011–2014. (NCHS CDC Data Brief Number 283). National Center for Health Statistics; online. https://www.cdc.gov/nchs/products/databriefs/db283.htm
Public Health England. (2020, December 3). Prescribed medicines review: Summary. GOV.UK. https://www.gov.uk/government/publications/prescribed-medicines-review-report/prescribed-medicines-review-summary
Research and Markets. (April 2021). Global Antidepressants Market (2020 to 2030)—COVID-19 Implications and Growth. Research and Markets. https://www.researchandmarkets.com/reports/5314992/antidepressants-global-market-report-2021-covid#pos-0
Shatan, C. (1966). Withdrawal symptoms after abrupt termination of imipramine. Canadian Psychiatric Association Journal, 11 Suppl, Suppl:150-158. https://doi.org/10.1177/070674376601101s27
Warner, C. H., Bobo, W., Warner, C., Reid, S., & Rachal, J. (2006). Antidepressant Discontinuation Syndrome. Am Fam Physician, 74(3), 449–456. https://www.aafp.org/afp/2006/0801/p449.html
Photo credits
- Review infographics courtesy of Cochrane Australia
- Photo by Kelly Sikkema on Unsplash

Excellent work, Adele. “With unrecognised withdrawal symptoms confounding the evidence base of antidepressant discontinuation, we cannot assume that what happens when people go off antidepressants is relapse. Therefore, justification for continuation may be shaky.” This really identifies the crux of the important research findings and the implications for paradigm shifts within conventional protocols. Insights that benefit clinicians and subsequently, patients, profoundly.